Living with Hemophilia: Coping Strategies and Quality of Life
Hemophilia Disease Overview:
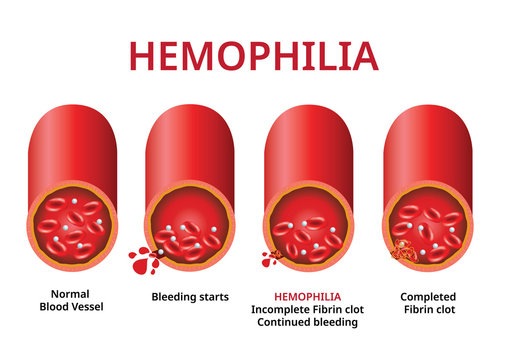
Hemophilia Disease is a rare but serious bleeding disorder that affects thousands of people worldwide. In this article, we will explore various aspects of Hemophilia disease, including diagnostic analysis, treatment options, the regulatory framework surrounding Hemophilia, competitive analysis, market trends, clinical trial assessment, and a conclusion that encapsulates the current state of Hemophilia research and care.
The Market Competitors Listed Below are Revolutionizing Healthcare with Innovative Inventions:
Factor Replacement Therapy
· Bayer
· Baxalta
· Novo Nordisk
· Emergent BioSolutions
· Catalyst Biosciences
· OPKO Health
Non-Factor Replacement Therapy
· Pfizer
· Spark Therapeutics
· Biogen
· Chameleon Biosciences
· Asklepios BioPharmaceutical
· uniQure
Gene Therapy
· CSL Behring
· BioMarin Pharmaceutical
· Alnylam Pharmaceuticals
· Dimension Therapeutics
· GeneVentiv
· Sanofi
Hemophilia Diagnostic Analysis:
Diagnosing Hemophilia is a critical first step in managing the disease effectively. Physicians rely on a combination of clinical assessments, medical history, and laboratory tests to diagnose Hemophilia. The two most common types, Hemophilia A and Hemophilia B, can be diagnosed through specific blood tests measuring the levels of clotting factors VIII and IX, respectively. Genetic testing can also identify specific mutations responsible for Hemophilia.
Hemophilia Treatment Analysis:
Treatment for Hemophilia primarily involves replacement therapy, where the missing clotting factor (VIII or IX) is infused into the patient’s bloodstream. This therapy can be administered as a preventive measure or as an on-demand treatment for bleeding episodes. Advances in treatment methods, such as extended half-life factor products and gene therapy, have significantly improved the quality of life for Hemophilia patients. These innovations offer longer lasting and more convenient treatment options, reducing the frequency of infusions.
Browse More Information:
https://www.diseaselandscape.com/genetic/hemophilia-disease-regulatory-insights
Regulatory Framework for Hemophilia:
The regulatory framework surrounding Hemophilia varies by region but typically involves government agencies like the Food and Drug Administration (FDA) in the United States. These agencies ensure the safety and efficacy of Hemophilia treatments, conducting rigorous evaluations before granting approvals. The framework is designed to safeguard patient interests and promote the development of cutting-edge therapies.
Competitive Analysis:
The Hemophilia treatment market is highly competitive, with several pharmaceutical companies producing clotting factor concentrates. Major players in this space include companies like Novo Nordisk, Shire (now part of Takeda), and CSL Behring. The competition has led to innovation and the development of new treatments and delivery methods, providing patients with a wider range of options, and improving their overall quality of life.
Market Trends:
The Hemophilia market has witnessed significant trends in recent years. These include:
- Gene Therapy: Research into gene therapy has gained momentum, potentially offering a one-time, curative solution for Hemophilia patients.
- Extended Half-life Products: The development of extended half-life factor products has reduced treatment frequency, improving patient adherence and convenience.
- Home-based Treatment: An increasing emphasis on home-based treatment allows patients to manage their condition more independently.
- Global Expansion: The Hemophilia treatment market has expanded globally, ensuring a broader reach of life-saving therapies.
Clinical Trial Assessment:
Clinical trials play a pivotal role in advancing Hemophilia care. Ongoing research and clinical trials explore new treatment options, improve existing therapies, and evaluate long-term safety. Patients with Hemophilia often participate in these trials, contributing to scientific understanding and helping to bring innovative treatments to the market.
Conclusion:
Hemophilia is a challenging disease that has seen remarkable progress in diagnosis and treatment. The combination of diagnostic advances, cutting-edge therapies, and a competitive market has significantly improved the quality of life for Hemophilia patients. The future holds promise with gene therapy and extended half-life products on the horizon, offering potential curative solutions. Regulatory agencies and clinical trials continue to play a crucial role in ensuring the safety and effectiveness of these treatments. As research and innovation continue, the outlook for Hemophilia patients is increasingly positive, providing hope for a brighter future.
Browse Through More Genetic Diseases Research Reports
Confronting Solid Tumors: Understanding the Challenges and Solutions
Spinal Muscular Atrophy (SMA): Unveiling the Road to Hope and Treatment
Understanding Cystic Fibrosis: Diagnosis, Treatment, Market Trends, and Future Prospects
Duchenne Muscular Dystrophy: The Battle for Strength and Hope